PharmaOS
The pharmacovigilance platform for reporting, monitoring and assessing patient safety data
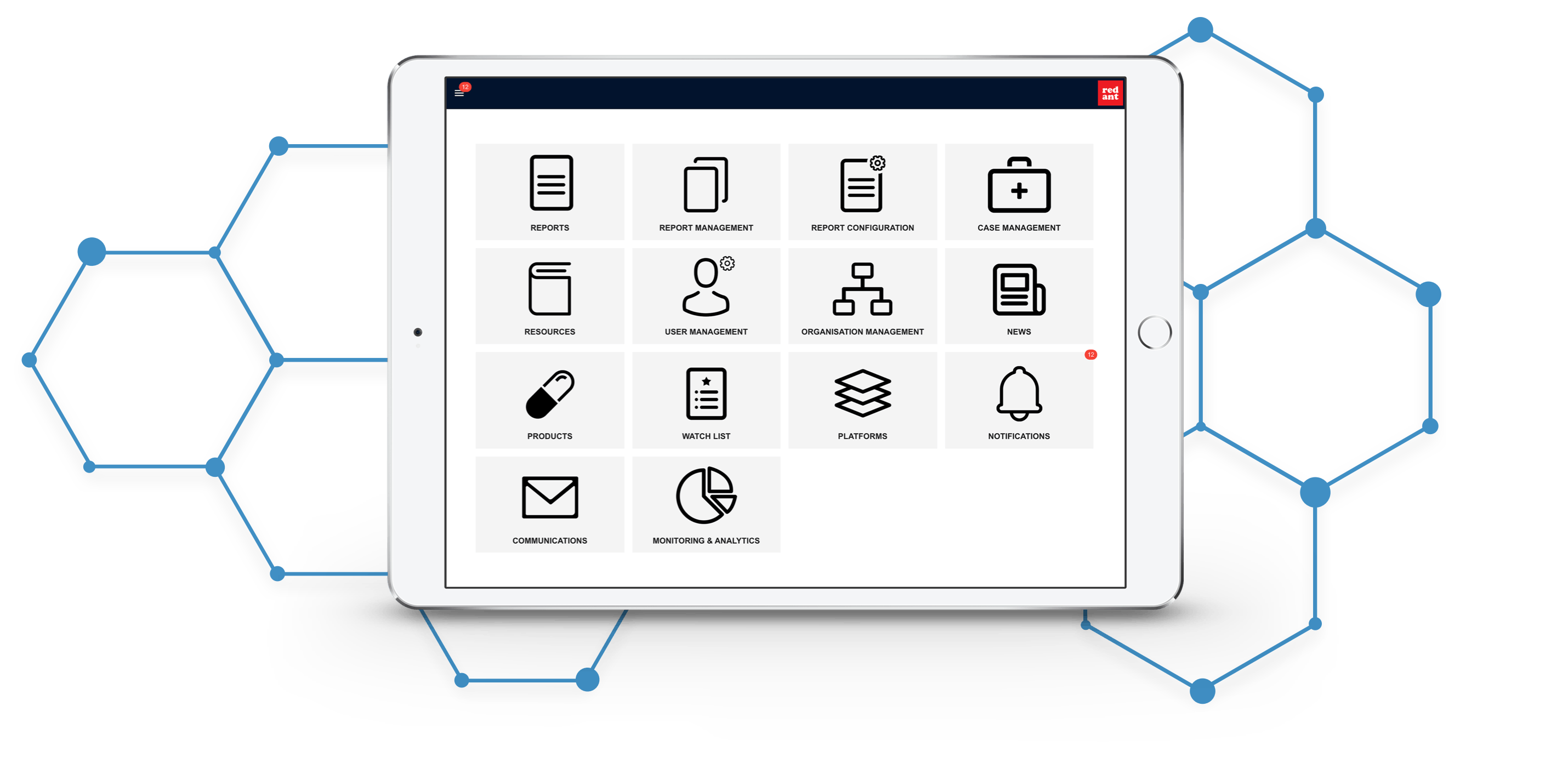
A powerful report management platform
PharmaOS is designed to support pharmaceutical companies' patient trials and new product launches to collect and manage patient feedback, adverse reaction reporting and patient follow-ups. It offers a comprehensive range of features, including:
Reporting forms configuration, designed for ease of use
Case management and handling
Analytics reporting
Organisation, platform and product library management
Notifications and alerts, with the ability to customise communication content
Case study – MHRA
Working together for 20+ years
The MHRA (Medicines and Healthcare products Regulatory Agency) regulates medicines, medical devices and blood components for transfusion in the UK, and has partnered with Red Ant for more than 20 years.


Red Ant's solution
Design, build and maintenance of a single pharmacovigilance platform for multiple organisations
Easy to use interface for the Yellow Card reporting system, which was vitally important during Covid for reporting on specialist medical equipment and the introduction of the vaccine
Supports use by multiple partners without the need for additional development work for each partner
Modular setup for partners to use in different applications and programmes
Allows partners to receive adverse drug reaction reports made via a variety of channels
Find out more about PharmaOS